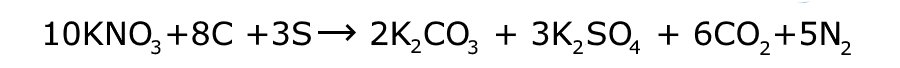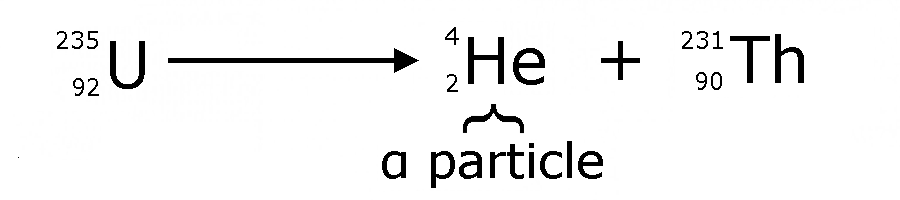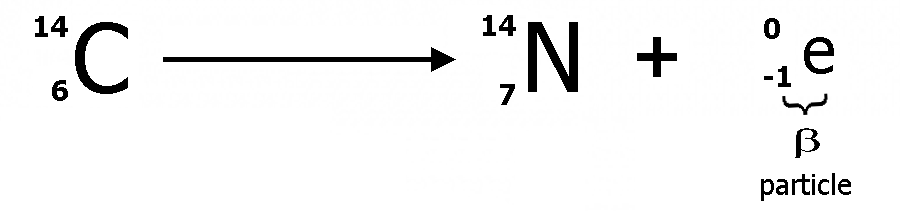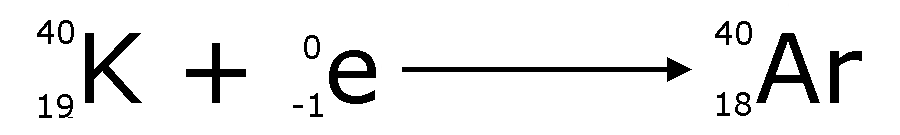Nuclear Weapons
Donald J. Trump Explains Uranium
To fully understand the Cold War,
one needs to understand the nature of the threats that faced the
world, chief among them were nuclear weapons. That is where this
page is centred.
Explosions
The term explosion is a general one for an accelerated release of energy generating extreme temperatures, releasing gases that expand in volume. Broadly speaking there are three kinds of explosion, physical, chemical and nuclear. A physical explosion is one when there is a sudden release of mechanical energy, such as the discharge of a compressed gas. These types of physical explosions include vessel rupture and rapid phase transition explosion, a simple example is the popping of corn! Chemical explosions require a chemical reaction, which could be a combustion or reaction (rapid exothermic reaction or rapid release of heat). Chemical explosions can occur in either vapour, liquid, or solid phases. A nuclear explosion involves changes to the nucleus of atoms, either by the splitting (fission) of nuclei, or the fusion of nuclei. We will not be concerned with physical explosions.
Chemical Explosions
Chemical explosions may be supersonic as in the case with detonations of high explosives like Pentaerythritol tetranitrate [C(CH2ONO2)4] (PETN), or subsonic and initiated by deflagration (combusting materials via heat transfer) of low explosives like gunpowder as seen in fireworks. There are many chemical reactions that will release energy. They are known as exothermic reactions. If the reaction proceeds slowly, the released energy will be dissipated and there will be few noticeable effects other than an increase in temperature. On the other hand, if the reaction proceeds very rapidly, then the heat energy will not be dissipated. Thus, a great quantity of energy can be deposited in a relatively small volume, then manifest itself by a rapid expansion of hot gases, which in turn can create a shock-wave or project fragments or a projectile outwards at high speed. Chemical explosions may be distinguished from other exothermic reactions by their extreme rapidity.
Most chemical explosions involve a limited set of simple reactions, all of which involve oxidation (reaction with oxygen or another oxidiser such as chlorine).
An explosion consists of an event releasing a large amount of energy in a short time-span. Broadly speaking energy can be described as potential or kinetic. Potential energy is energy of configuration, position, or the capacity to do work. For example, the relatively unstable bonds among the atoms that comprise 2,4,6-trinitrotoluene [C6H2(NO2)3CH3] (TNT), possess chemical potential energy. Potential energy can, under suitable conditions, be transformed into kinetic energy, which is energy of motion. When a conventional explosive such as TNT is detonated, the unstable chemical bonds are converted into bonds that are more stable, producing kinetic energy in the form of blast and thermal energies. This process of transforming a chemical system's bonds from lesser to greater stability is exothermic, in other words it is a reaction which produces heat energy.
The first chemical explosive was gunpowder, in which the fuel is a mixture of sulfur and carbon, with potassium nitrate as the oxidiser. It was invented in China in the 9th century CE. Initially it contained too small a concentration of potassium nitrate (about 25%). In the middle of the following century the first formulary for gunpowder and its uses was published for the military. By this time the highest level of potassium nitrate had risen to about 50%, still not an explosive. However, by 1280 explosive gunpowder was in use, with a composition of 75% potassium nitrate, 15% carbon and 10% sulfur. In that year the biggest man-made explosion for hundreds of years occurred at Wanggongchang , when stored gunpowder exploded, killing 100 guards.
It was three hundred years before
any significant advance was made in the world of explosives, and
that was merely the replacement of potassium nitrate with the
cheaper, though not so good, sodium nitrate (Chile saltpeter). The
sodium nitrate version was fine for mining, but not for military
purposes, the reason being that sodium nitrate is deliquescent,
meaning that it absorbs water from the air to the extent of
dissolving, meaning it cannot be stored.
The first new explosive was nitroglycerine [C3H5(ONO2)3], synthesised by the Italian chemist Ascanio Sobrero, in 1847. He considered it too dangerous to be of any practical use, and is quoted to have said: "When I think of all the victims killed during nitroglycerine explosions, and the terrible havoc that has been wreaked, which in all probability will continue to occur in the future, I am almost ashamed to admit to be its discoverer." Various improvements were made using a variety of additives, the most famous being kieselguhr (diatomaceous earth), forming Dynamite, and invented by Alfred Nobel in 1867. By the end of WWII a number of other high explosives had been developed, but none of them came anywhere near the power of nuclear explosives.
Atoms and Elements
The idea of atoms is not a new on, it is a very old idea, appearing in several ancient cultures, including those of Greece and India.The word atmos was coined by the Greeks Leucippus and Democritus in about 450 bce. However these beliefs were based on metaphysical reasoning, rather than science The idea disappeared until the 18th Century when they were brought up by John Dalton (1766-1884). His theory can be summarised as follows:
- Elements are made up of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass, and other properties.
- Atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be subdivided, created, or destroyed.
- Atoms of different elements combine in simple, whole-number ratios e.g. 1:1, 1:2, 2:3, 1:3:3, and so on to form compounds
- In chemical reactions, atoms
are combined, separated, or arranged.
* Some of these have had to be modified as a result of later discoveries, but broadly speaking they are still true.
There are just 94 naturally occurring elements an a couple of dozen or so that have been created artificially.
In the right hand column you will see a simplified equation for the detonation of gunpowder. In it you will note that the numbers of each type of atom on the left of the arrow are the same as those on the right. This is a characteristic of a chemical reaction, the atoms are not themselves changed, they are simply re-arranged. The properties of the substances on the left are different to those on the right. On the left there are three solids (at room temperature), on the right they have become two solids and two gases. These gases take up much more volume than the solids, magnified by the fact that they are also at a very much higher temperature.
Atomic Particles and Structure
The electron was the first atomic
particle to be discovered by J.J.
Thomson in 1897. The proton was discovered by Ernest
Rutherford in 1917, shortly after he had demonstrated the
existence of the nucleus. It was not until 1932 that James
Chadwick discovered the neutron.
Rutherford-Bohr atomic model
The Rutherford-Bohr atomic model
represents the atom by indicating the number of protons in the
nucleus as well as the number of electrons in each electron shell
(also called energy levels or orbitals). The model was developed by
Niels Bohr, based upon the earlier work of Ernest Rutherford, they
were working together at the time.
Atomic facts
- An atom of an element is the smallest particle of the element which retains its chemical properties.
- The neutron and proton weigh approximately the same (1 atomic mass unit or amu).
- The electron is much smaller with a mass of 0.00054858 amu.
- Atoms are too small to be seen under a light microscope.
- Most of the mass of the atom is in the core of the atom known as the nucleus.
- A nucleus always has one or more positive electrical charges.
- Electrons have a negative charge equal but opposite to that of protons.
- Electrons move around the nucleus at relatively large distances.
- Most of the volume of an atom is empty space.
- The chemical properties of each element depend upon the number of protons in their nucleus.
Periodic Table of the Elements
The elements may be listed in order of the number of protons (their atomic number). Elements in a vertical column are said to be in the same group, and have similar chemical properties.
Isotopes
Isotopes are variants of a particular chemical element which differ in neutron number, and consequently in nucleon number. All isotopes of a given element have the same number of protons but different numbers of neutrons for each isotope.
The number of protons within an atom's nucleus is called it's atomic number (Z) and is equal to the number of electrons in the atom, this means that an atom is electrically neutral. Each atomic number identifies a specific element, but not an isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number (A), and each isotope of a given element has a different mass number.
Radioactive Decay
Radioactive decay is a complex matter, rather than a single thing it is a number of processes. Certain atoms are unstable or radioactive, in fact it is a property of the nuclei of those atoms. In radioactive decay the nucleus emits sub-atomic particles and/or energy in the form of gamma rays. The SI unit for measuring radioactive decay is the Becquerel (Bq), formerly it was the Curie (Ci). If a quantity of radioactive material produces one decay even pre second, it has a an activity of one Bq. Since any reasonably sized sample of radioactive material contains many atoms, a becquerel is a tiny amount of activity. The americium source in a modern ionisation type smoke detector has an activity of about 37kBq, and that is due to about 300mg of the isotope.
The most common decay modes are alpha, beta and gamma. In alpha decay the emitted particle is an alpha particle consisting of two protons and two neutrons, equivalent to a helium nucleus. In beta decay the emitted particle is an electron. In the case of gamma decay energy is emitted in the form of gamma ray photons.
The neutrons and protons in a nucleus have various interactions. The strong nuclear force is the most powerful force over subatomic distances. The electrostatic force is also significant. Also of importance is the weak force. The interplay of these forces are very complex. If the configuration of the particles in a nucleus changes even very slightly, the particles can possibly fall into a lower-energy arrangement, resulting in spontaneous nuclear decay. The resulting transformation changes the structure of the nucleus, it is a nuclear reaction, in contrast to chemical reactions where the nucleus does not change.
Some nuclear reactions involve external sources of energy in the form of collisions from nuclear particles, however these are not considered decay.
The decay process is entirely
random, it is not possible to predict when a particular nucleus will
decay. Different radionuclides decay at different rates, commonly
described in terms of half-lives. Given a sample of a particular
radioisotope, the half life is the time taken for half of the nuclei
to decay. Half lives vary from as long as 109 years to
10-6 seconds.
α decay (alpha decay)
Alpha decay is a form of
radioactive decay in which an atomic nucleus characterised by mass
number A, and atomic number Z ejects an alpha particle, which is a
4He nucleus, and transforms into a nucleus with mass number that is
reduced by 4, and atomic number reduced by 2.
β decay (beta decay)
Beta decay (sometimes called neutron decay) is a type of radioactive decay in which a ?-particle is emitted. A neutron in space will only last about ten minutes before ejecting an electron and leaving behind a proton. This is energetically possible because the mass of the neutron exceeds sum of the masses of the proton and electron (this is part of Einstein's famous E= mC2 equation). Heavier nuclei have more neutrons than protons, because the Coulomb repulsion makes it harder to bind protons. Coulomb repulsion is the repulsive force between two similar charges., as described in Coulomb's Law. But neutrons are fermions and the Pauli exclusion principle requires that as we add more and more neutrons, the energy of the added neutrons will be higher and higher. So β-decay is favoured energetically.
In ?-decay the weak nuclear interaction converts a neutron into a proton while emitting an electron and an anti-neutrino. The decay process transmutes one element into another.
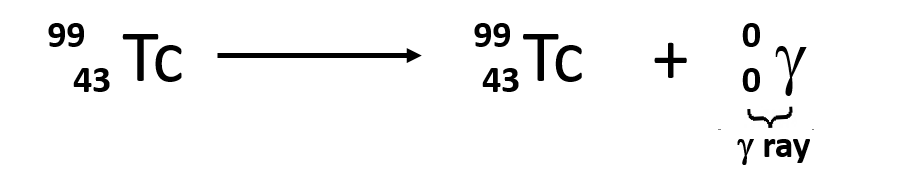
γ decay (gamma decay)
Emission of γ rays (gamma rays) is
similar to emission of light photons by excited states of atoms. The
nucleus can be excited by having just emitted an α- or β-particle,
or by colliding with another nucleus, or by being bombarded by
neutrons. All these events can lead to a nucleus which is excited,
and electromagnetic radiation is emitted.
Photons with energies of more than
10keV are frequently called γ-rays,
although electromagnetic radiation from around 10keV to several
hundred keV is also referred to as hard x-rays. There is no physical
difference between γ-rays and x-rays
of the same energy, these are two names for the same electromagnetic
radiation. Gamma rays are distinguished by their origin.
Sometimes the radioactive decay can result in the result of multiple forms of radioactive emission, for example in the decay of radon-222 (222Rn), both α- or β-particles are emitted as well as γ-rays.
Positron emission (ε+ decay)
Positron emission happens with nuclides in which the neutron:proton ratio is low. Positron decay is the conversion of a proton into a neutron with the emission of a positron. The neutron:proton ratio increases, and the daughter nuclide is more stable than the parent nuclide. The positron has the mass of an electron, but a positive electrical charge. Thus, the overall mass of the nuclide doesn't change, but the atomic number is decreased by one, which causes a change in the elemental identity of the daughter isotope.
Electron capture
Electron capture occurs when one of the inner electrons in an atom is captured by the atom's nucleus. When it occurs an inner shell electron combines with a proton, forming a neutron.
Nuclear fission
The element uranium as found in
nature is a mixture of isotopes but most of it consists of atoms
with 92 protons and 146 neutrons, i.e. a total of 238 mass units in
each nucleus: hence this isotope is referred to as 238U
or U-238. Another isotope, 235U, with 92 protons and 143
neutrons is found in natural uranium to the extent of 0.7%. 235U
is used in many nuclear reactors and in some nuclear weapons when
much of the 238U has been removed, this is process is
known as enrichment, and the product is known as enriched uranium. 238U
is not fissile, but 235U is, fissile material is that
which is capable of sustaining a nuclear fission chain reaction.
Weapons grade uranium is enriched to better than 90%.
Plutonium is the first of the transuranic elements to have been made, transuranics have atomic numbers greater than that of uranium. 239Pu (94 protons plus 145 neutrons) is produced either in a nuclear reactor from 238U or in a cyclotron. Using similar methods 233U can be produced from thorium. 239Pu and 233U are both fissile, and like 235U can be used as nuclear explosives.
The isotopes 233U, 235U,
and 239Pu are radioactive and their atoms disintegrate
in the various ways mentioned above. There is another way in which
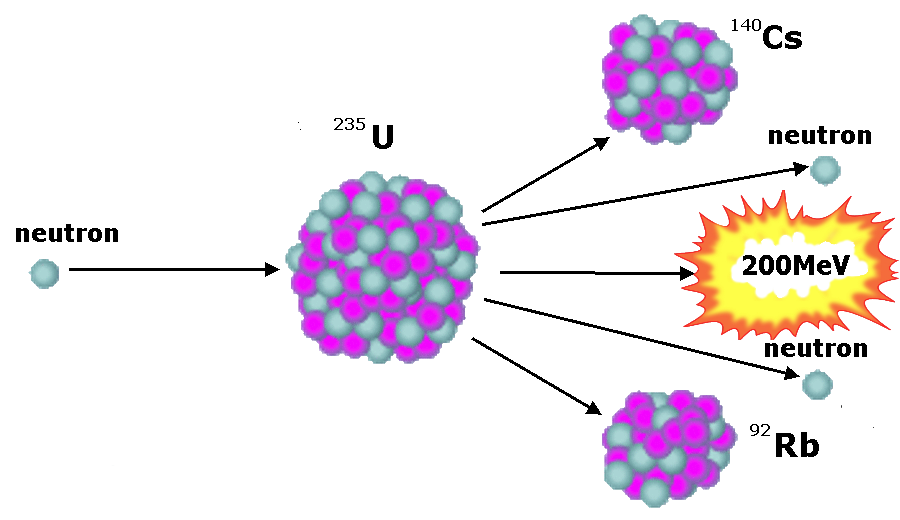
these atoms can break up. If their nuclei capture, or are hit by, a
neutron each nucleus splits up into two not quite equal fragments.
At the same time one to three neutrons are released. The fissile
charge, even in a small nuclear weapon, even though it may weigh
only a few kilos, contains multiple billions of atoms and these do
not all split up in the same way, causing multiple different
daughter products. One of the first described fission process,
involved 235U splitting into 90Kr (krypton)
and 144Ba (barium). The products of typical fission
detonations contain about 200 different radioisotopes of about 35
elements.
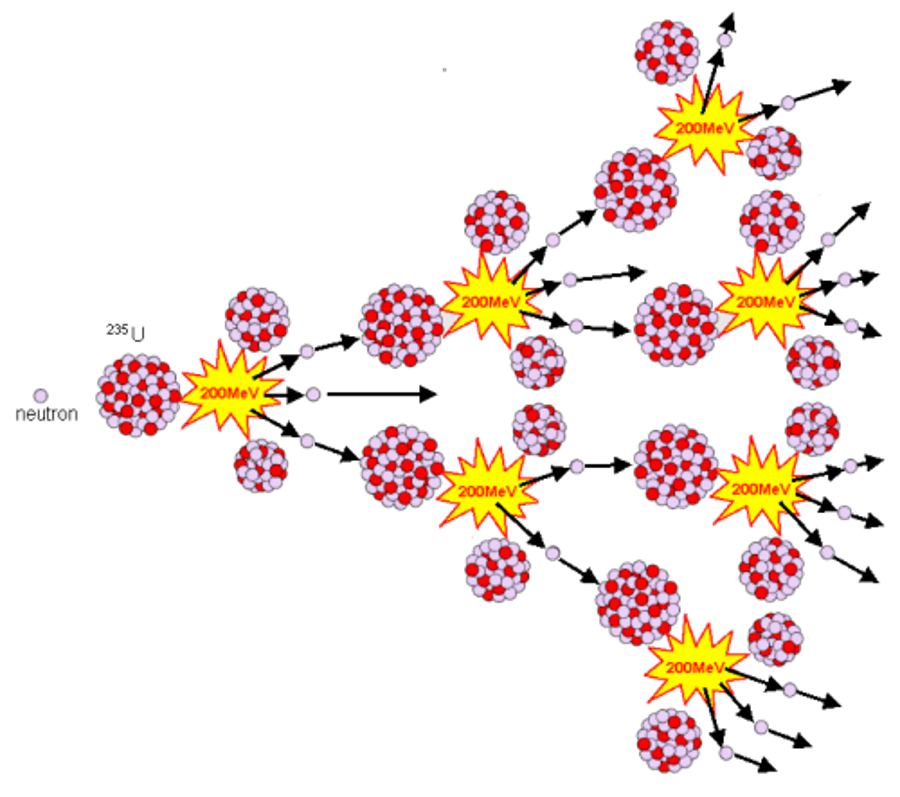
Chain reactions
If more than two neutrons are
released during a decay, then a chain reaction may occur, given that
other conditions are favourable. This was first suggested by Leo
Szilard. in the case of 235U the number of neutrons
released is 2.3 on average.
Critical sizes of fissile charges
When a piece of fissile material is below a certain critical size a few of the atoms are continually undergoing fission, but more neutrons escape from the surface than are produced in fission and an increasing chain of fissions is not built up. If several pieces of fissile material, totaling more than the critical amount, are brought together inside a strong container, or tamper, a nuclear detonation results. The critical size depends upon a number of factors, including the element and which isotope is involved, the purity of the material, its density, and the nature of the container and whether it absorbs or reflects neutrons. Commonly you will hear the term "critical" mass, this is a fundamental error in thinking, derived from over-simplification this is even evident in Serber's "Los Alomos Primer". It is not a mass that is critical, it is a combination of factors. Early experiments during the Manhattan Project. This was a particular problem with plutonium, as some samples were of one allotropic form and some another. There are six allotropes of 239Pu with densities from 16.51 - 19.86g/cm3. Purity of the desired isotopes is also a major problem, even small amounts of impurities lead to major changes in fissile properties of the material, generally because they absorb neutrons without fissioning.
If critical mass means anything, it refers to a perfect sphere of uniform density in specific conditions. The critical mass of 235U is reported to be 52kg for an unconfined sphere of the pure metal and would be about 170mm in diameter. The mechanical complication of bringing together, rapidly and simultaneously, a number of sub-critical pieces of fissile material sets a practical limit to the size of nuclear fission weapons.
Each fission releases energy, in
the case of 235U this is 202.5MeV. This may not sound
much, but each kilogram completely fissioned would release 82TJ of
energy.
Nuclear fission weapons
There are two principal types of
fission weapon, the gun type and the implosion type, the second
being considerably more complex.
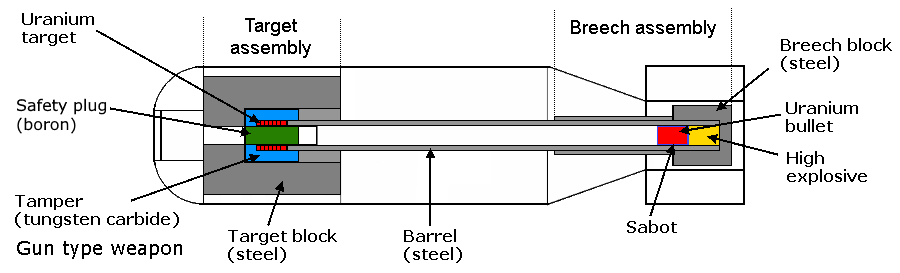
Gun type weapon
In the gun type weapon two pieces
of fissionable material, each rather more than half sub-critical are
used, one is designated the "bullet" and the other the
"target". When brought together they form a super-critical
quantity. The bullet is fired down a "barrel" by conventional high
explosive. The casing of the weapon is extremely strong, the idea
being to contain the force of the explosion until it builds to the
desired degree. Various versions of this type have been made, the
earliest British form used two hemispheres, minimising the quantity
of fissionable material required to achieve super-criticality. The
picture shown is of an alternative type with a hollow target, it is
simpler, but requires some 30% more fissionable material. This
latter form was that used in the Hiroshima bomb.
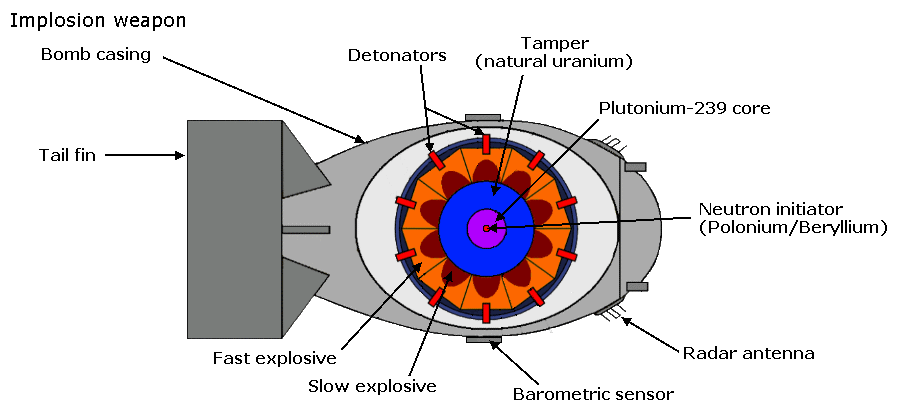
Implosion type weapon
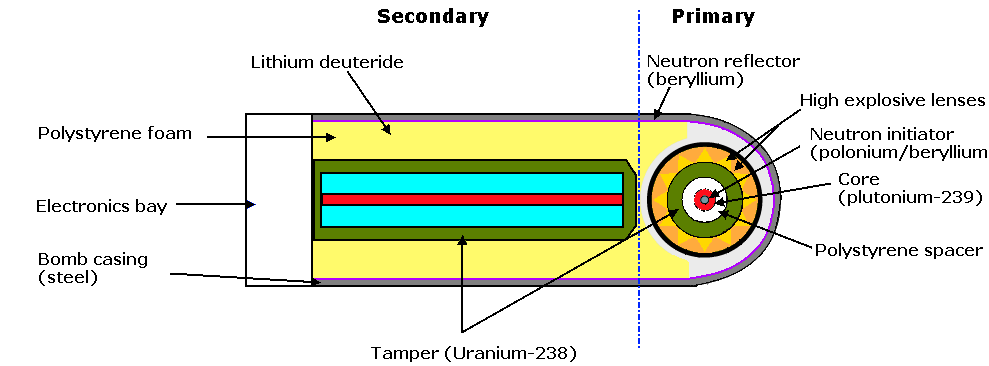
The gun type weapon can only used 235U as 239Pu suffers from a condition known as pre-detonation, in other words it will start a chain reaction prior to becoming fully assembled into the super-critical mass, and produce a far lower level of energy than is required. The pre-detonation is due to the presence of 240Pu contamination. Manhattan Project scientists devised the implosion method of assembly, in which high explosives are arranged to form an imploding shock wave which compresses the fissile material to super-criticality. When the high explosive is detonated, an inwardly directed implosion wave is produced. This wave acts upon the heavy metal tamper which in turn compresses the sphere of fissionable material. The decrease in surface to volume ratio of this compressed mass plus its increased density is then such as to make the mass super-critical. In more recent weapons the fissionable material is usually surrounded by a tamper consisting of 238U. The HE is exploded by detonators timed electronically by a fusing system, which may use altitude sensors or other means of control. The design of the explosive charges and their firing is complex and is was a major issue for the Manhattan Project engineers. Specialised components had to be developed to construct the firing circuitry. The bomb which was used for the Trinity test and for the bomb dropped on Nagasaki. In the centre of the fissile mass is a source of neutrons, usually composed of beryllium and polonium, known as the initiator.
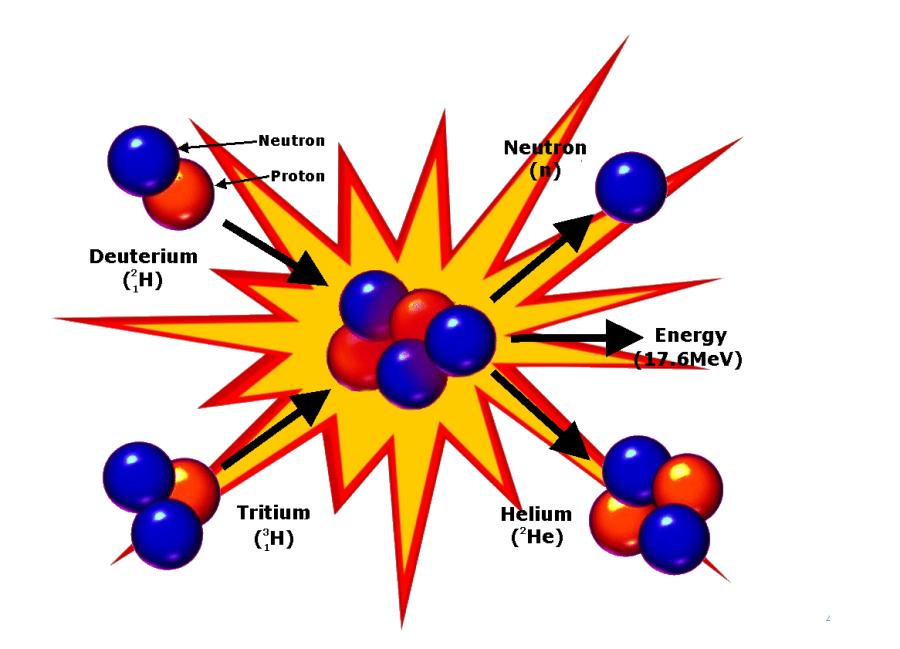
Nuclear fusion and thermonuclear weapons (H-bombs)
A temperature of many million
degrees Celsius is reached in the detonation of a nuclear fission
weapon. At this temperature atoms are stripped of most of their
surrounding cloud of electrons and the nuclei move at very high
speeds experiencing many collisions with one another. Under these
circumstances the nuclei of the rarer hydrogen isotopes deuterium
and tritium have enough kinetic energy to overcome the repulsive
forces between their single positive charges, and they are able to
fuse together. The energy released in the fusion is about
one-twelfth of that released in the fission of a single 235U
nucleus, but on an equal weight basis, the fusion energy is about
2.5 times as large as the fission of 235U. At one time
it was thought that the deuterium and tritium would have to be
liquified by refrigeration. In fact the first fusion bomb exploded
by the Americans in 1952, was like this, it was too massive ever to
have been used as a weapon. Later weapons used lithium deuteride
(LiH, sometimes written LiD), a white powder, sometimes formed into
a ceramic material. Each neutron (1 atomic mass unit) released by
the triggering fission component splits a lithium atom (6 mass
units) into helium (4 mass units) that takes no further part
in the reaction, and tritium (3 mass units) which then fuses
with a deuterium nucleus.
There is no limit, other than the
convenience of delivery, to the size of a fusion weapon, in fact the
largest weapon detonated was the Soviet 50MT Tsar-Bomba,
although they claimed to have produced a 100MT device. Helium gas,
the main product of fusion detonation, is not radioactive, but the
high speed neutrons produced collide with atoms of other substances,
including nitrogen in the air, in so doing an intense burst of
γ-radiation, commonly known as gamma flash. The neutrons may also
induce radioactivity in some of the ground material if detonated at
low enough level, they also induce radioactivity in bomb components.
In the process of fusion a neutron is released at very high velocity, from each pair of reacting nuclei and it has enough energy to split the atoms of the more common isotope of uranium, 238U. Thus, if 238U metal is used for the bomb case, it will act not only as a tamper but will increase the amount of fission products. This is known as a fission-fusion-fission or dirty bomb.
The bomb illustration is of the Teller-Ulam configuration. There are a number of other configurations possible, and modern weapons are commonly more-or-less spherical.
Neutron bomb
The name neutron bomb is a
misnomer created by Walter Pincus of the Washington Post, in the
summer of 1977, and then applied to the W79 and W70-3 warheads for
the M1 8-inch gun, and lance short range ballistic missile. The
correct terminology for these warheads is "reduced blast/enhanced
radiation (RB/ER) warheads". All that is necessary to produce an
RB/HR weapon is to remove the 238U tamper and the
neutron reflector from a small thermonuclear device. This results in
a weapon with a reduced blast effect but enhanced radiation, both
neutrons and γ-radiation. Its blast and heat effects would be
confined to an area of only a few hundred metres in radius, but the
radiation effects would extend to a larger radius of 1,000 to 2,000
metres. High energy neutrons, short lived, could penetrate armour or
several metres of earth and concrete and would be extremely
destructive to living tissue. Because of its short-range
destructiveness and the absence of long-range effects, such a weapon
might be highly effective against tanks and infantry formations, but
might not endanger nearby cities or other population centres.
Although several countries, including the USA, USSR, France and
possibly Israel, developed such weapons it is believed that no
country now includes them in their arsenals.