Nuclear radiation
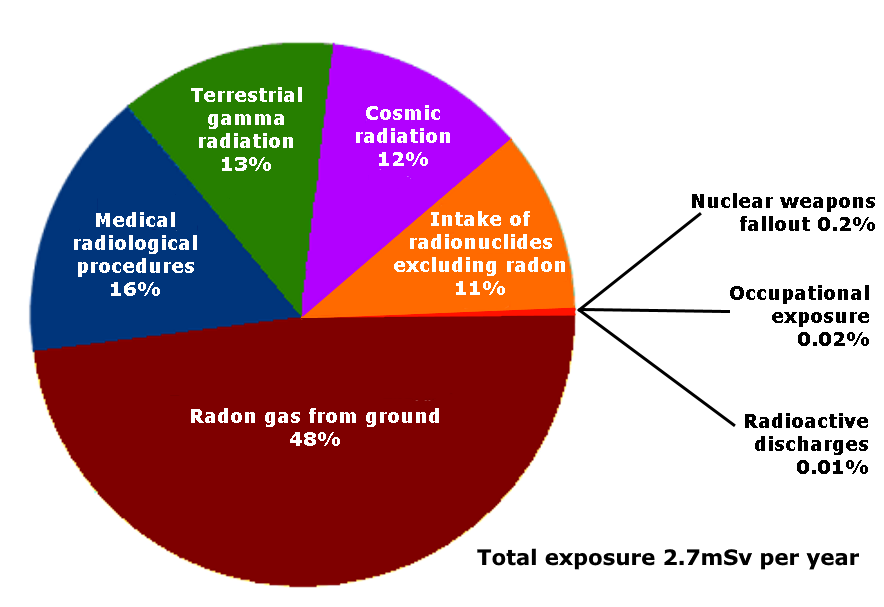
We are all subject to radioactivity; depending on lifestyle, and where we live, the amount varies. Sources of radiation in the environment comprise radiation from the underlying geology, cosmic radiation, industrial pollution, fallout from nuclear testing, radiological medical examinations and from the food we eat. Ionizing radiation is emitted when radioactive substances decay. Radioactive decay is the spontaneous breakdown of an atomic nucleus resulting in the release of energy and/or matter from the nucleus. A radioactive substance (isotope) has unstable nuclei that do not have enough binding energy to hold the nucleus together. Radioisotopes tend to lose matter and energy to become stable isotopes so they are constantly changing in order to stabilize. In the process, they will release energy and matter from their nucleus and sometimes transform into a new element. This process, called transmutation, is the change of one element into another as a result of changes within the nucleus. The radioactive decay and transmutation process will continue until a new isotope of an element is formed that has a stable nucleus and is not radioactive.
Ionizing radiation
Ionizing radiation is emitted when radioactive substances decay. Radioactive decay is the spontaneous breakdown of an atomic nucleus resulting in the release of energy and/or matter from the nucleus. A radioactive substance (isotope) has unstable nuclei that do not have enough binding energy to hold the nucleus together. Radioisotopes tend to lose matter and energy to become stable isotopes so they are constantly changing in order to stabilize. In the process, they will release energy and matter from their nucleus and often transform into a new element. This process, called transmutation, is the change of one element into another as a result of changes within the nucleus. The radioactive decay and transmutation process will continue until a new isotope of an element is formed that has a stable nucleus and is not radioactive. Radioactive decay results in ionising radiation.
Ionizing radiation is radiation that has enough energy to remove electrons from atoms or molecules (groups of atoms combined together) when it passes through or collides with material. The loss of an electron with its negative charge causes the atom (or molecule) to become positively charged. The loss (or gain) of an electron is called ionization and a charged atom (or molecule) is called an ion.
NB: Microwave, infrared (IR) and ultra-violet (UV) radiation are examples of non-ionizing radiation. Non-ionizing radiation does not have enough energy to remove electrons from atoms.
We are exposed to ionizing radiation from natural sources in two ways:
- We are surrounded by naturally-occurring radioactive elements in the soil , we are subjected to cosmic rays entering the earth's atmosphere from outer space.
- We receive internal exposure from radioactive elements which we take into our bodies through food and water, and through the air we breathe. In addition, we have radioactive elements (Potassium 40, Carbon 14, Radium 226) in our blood or bones.
There are four types of ionising radiation associated with nuclear weapons:
- α (alpha) particles,
- β (beta) particles,
- γ (gamma) rays,
- n (neutron) particles
Alpha particles
An alpha particle consists of two protons and two neutrons, the equivalent of the nucleus of a helium atom. Alpha particles readily ionize material they contact and transfer energy to that material's electrons. An alpha particle can travel several millimetres in air, but its range decreases with increasing density of the medium. Alpha particles are blocked by the thinnest layer of solid material, even paper. They are intensely ionising, and whilst they cannot penetrate even the dead outer layers of the skin, if an alpha-emitter is ingested, breathed in, or penetrates the skin, then it can cause immense damage, including burning the tissues and may induce cancer. The mass of an alpha particle is 6.64 x 10-27 kg (0.00000000000000000000000000664 kg). None of the standard civil defence issue instruments could detect alpha radiation.
Due to the mechanism of their production, in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 5% the speed of light. They are a highly ionizing form of particle radiation, and, when resulting from radioactive alpha decay, have low penetration. They are able to be stopped by a few centimeters of air, or by the skin.
Long range alpha particles from fission are three times as energetic, and penetrate three times as far. The alpha particles from fallout products have a short range.
When alpha particle emitting isotopes are ingested, they are far more dangerous than their half-life or decay rate would suggest, due to the high relative biological effectiveness of alpha radiation to cause biological damage. Alpha radiation is, on average, about 20 times more dangerous, and in experiments with inhaled alpha emitters up to 1,000 times more dangerous, than an equivalent activity of beta emitting or gamma emitting radioisotopes. Alpha-emitters are not a significant hazard if outside of the body.
The relative proportion of alpha-emitters to beta and gamma-emitters in fallout is quite small.
Beta particles
Beta radiation is also particulate. A beta particle is an electron or a positron and is much lighter than an alpha particle, thus, it takes beta particles a longer distance than alpha particles to lose energy. A medium-energy beta particle travels about one metre in air and one millimetre in body tissue. The mass of a beta particle is 9.10 x 10-31 kg. Electrons and positrons each have a very small mass, so we don't expect an electron-volt to represent very much energy. One electron-volt is only 1.6 x 10-19 joules of energy, in other words, 0.16 billion-billionth of a joule. One joule (abbreviated J) is equivalent to the amount of energy used by a one-watt light bulb lit for one second. The energy associated with the radioactive decay ranges from thousands to millions of electron-volts per nucleus, which is why the decay of a single nucleus typically leads to a large number of ionizations.
Whenever beta particles are emitted by a nucleus, either a neutrino (when positron is emitted) or an antineutrino, when electron is emitted, is also emitted, but neutrinos/antineutrinos don't interact with matter and are not of significance from a radiation point of view. When an electron is emitted, a neutron in the nucleus is transformed into a proton; when a positron is emitted, a proton is transformed into a neutron. This transformation of a proton or neutron to a neutron or proton will result in a new nucleus in a lower energy state and this difference in energy accounts for the outgoing energy of the beta particle. The energy results in a velocity approaching that of light.
Beta radiation can be dangerous both internally and externally. The particles are stopped by moderately thick clothing or a few millimetres of aluminium, or a few centimetres of wood. Beta-emitters are most hazardous when they come into direct contact with the skin or if they are taken into the body.
Neutrons
Neutrons are neutral particles meaning that they have no electric charge, originating in the nucleus of atoms, they have very nearly the same mass as a proton. Unlike alpha and beta particles, they do not interact with electrons or cause ionization directly. Neutrons can, however, ionize indirectly in a variety of ways: elastic collisions, inelastic scattering, nonelastic scattering, capture reactions, or spallation processes. These processes variously result in the emission of gamma rays, beta radiation, and, in the case of spallation, more neutrons. Since neutrons carry no charge they are unaffected by the charges positive charges on nuclei or by the negative charges on electrons, they are only stopped or deflected by direct collision, which means they can travel considerable distances through the atmosphere, but are absorbed more quickly than gamma rays.
Gamma rays
Gamma rays are electromagnetic radiation, they have the shortest wavelength and greatest energy within the electromagnetic spectrum. A radioactive element may emit gamma rays, in discrete bundles, or quanta, called photons, if the nucleus remaining after alpha or beta decay is in an excited state. Gamma rays can penetrate much more deeply than alpha or beta particles; a high-energy gamma ray photon may pass through a human without interacting with tissue at all. When gamma rays interact with tissue, they ionize atoms.
Gamma rays, like x-rays are very penetrating electromagnetic radiation. Gamma-rays fall in the range of the EM spectrum above soft X-rays. Gamma-rays have frequencies greater than about 1019hertz (Hz) (10 exahertz), and wavelengths of less than 100 picometers (pm) a picometer is one-trillionth of a metre. They occupy the same region of the EM spectrum as hard X-rays. The only difference between them is their source: X-rays are produced by accelerating electrons which collide with a target, whereas gamma-rays are produced by atomic nuclei.
Fallout gives off gamma rays with a range from very soft and readily absorbed to very hard and penetrating. A relatively thin shield is able to absorb the very soft gamma rays, whereas it can take a great thickness of relatively dense material to stop the harder ones.
Measuring Radioactivity
Environmental and biological measurements of radioactivity are generally expressed as concentrations of radioactivity in soil, water, air, or tissue. Measurements are in becquerels per cubic metre or disintegrations per minute per 100 square centimetres. Sometimes, the weight of a radioactive material per unit of soil or tissue might be given and expressed in parts per million, or ppm, and can be expressed in terms of mass. This can be converted into radioactivity units, since we know the specific activities of various radionuclides. Disintegrations per minute per 100 square centimetres (dpm/100 cm2) is a unit commonly used to measure the surface contamination of an object, such as concrete or metal. Environmental and biological measurements may take into account the relative effects of different types and energies of radiation. Information about measuring instruments can be found on the RADIAC page.
Ionizing radiation is measured in terms of:
- the strength or radioactivity of the radiation source,
- the energy of the radiation,
- the level of radiation in the environment, and
- the radiation dose or the amount of radiation energy absorbed by the human body.
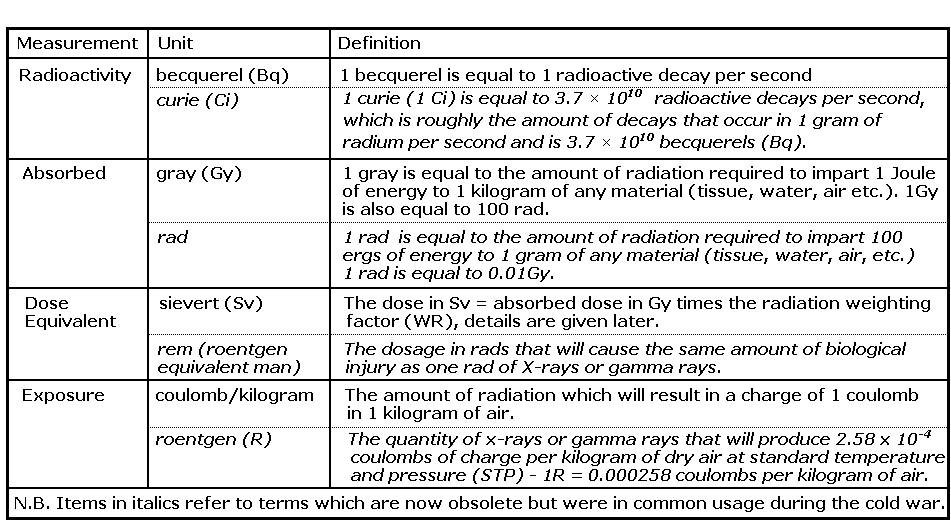
Ionizing radiation can be measured using units of electron volts, ergs, and joules. The electron-volt (abbreviated eV) is a unit of energy associated with moving electrons around. An electron is 'tightly bound' in a hydrogen atom (one proton and one electron). It takes energy to move this electron away from the proton, in fact it takes 13.6 electron-volts of energy to move this electron completely away from the proton. When that happens we say that the atom is 'ionized'. In the jargon, the 'ionization energy' of the tightly bound electron in hydrogen is 13.6 electron volts. Electrons have a very small mass, so we don't expect an electron-volt to represent very much energy. One electron-volt is only 1.6 x 10-19 joules of energy, in other words, 0.16 billion-billionth of a joule. One joule (abbreviated J) is equivalent to the amount of energy used by a one-watt light bulb lit for one second. The energy associated with the radioactive decay ranges from thousands to millions of electron-volts per nucleus, which is why the decay of a single nucleus typically leads to a large number of ionizations.
In the early days of civil defence in the UK the most commonly used radiation unit was the röntgen (R). The röntgen (R) was defined as that quantity of radiation which produces in l cm3 of air one unit of charge of either sign, thus de?ning a unit of exposure. Units of absorbed dose, the actual energy absorbed in the tissue being irradiated, are now used, in older texts the rad (radiation absorbed dose) and rem (röntgen equivalent man) may be found. The unit now used is the gray (Gy) which is equivalent to 100 rads (R). Different radiation types and energies have greater or lesser effect per unit dose, so they are all expressed relative to the effects of X-rays, i.e. a unit equivalent dose is used. To calculate the röntgen equivalent in man, the absorbed radiation dose is multiplied by a radiation weighting factor that is dependent on the type and energy of the radiation. The current SI unit of equivalent dose is the sievert (Sv). For X-rays and gamma rays the equivalent dose in sieverts and the absorbed radiation dose in grays are the same. The relationship between the different dose units is:
1 gray (Gy) - 1 joule/kg = 100 rads (R) = 100 rems (r) = l sievert (Sv) = 1,000 millisieverts (mSv) =1,000,000 microsieverts (?Sv)
Details of the working of radiation measuring equipment as used during the Cold War by the British civil defence services can be found on the RADIAC page.
Radiation units
Specific activity
Specific activity is a measure of the radioactivity of a unit weight of substance. The units are becquerels per gram. This allows us to compare whether a substance is more or less radioactive than another. The specific activity of a radionuclide is inversely proportional to its atomic weight and its half-life.
Environmental and biological measurements of radioactivity are generally expressed as concentrations of radioactivity in soil, water, air, or tissue. Measurements are in becquerels per cubic meter or disintegrations per minute per 100 square centimeters. Sometimes, the weight of a radioactive material per unit of soil or tissue might be given and expressed in parts per million, or ppm, can be expressed in terms of mass. This can be converted into radioactivity units, since we know the specific activities of various radionuclides. Disintegrations per minute per 100 square centimetres (dpm/100 cm2) is a unit commonly used to measure the surface contamination of an object, such as concrete or metal. Environmental and biological measurements may take into account the relative effects of different types and energies of radiation. Such measurements are an essential component of determining the safety, or otherwise, of foods and water, and also the advisability of growing crops or grazing livestock, following a nuclear attack. In the UK the specific activity of grass was measured routinely following the Windscale accident, and throughout the Cold War, by the Ministry of Agriculture, Fisheries and Food.
Dose
Placing a body near a radioactive source results in exposure. To evaluate the hazard from this exposure one must compute the absorbed dose. This is defined as the energy imparted to a defined mass of tissue. Dose is generally not uniform over the body. A radioactive substance can be selectively taken up by different organs or tissue, and some tissues and organs are more sensitive to the effects of radiation than others, for example tissues where there is rapid generation of cells such as those where blood cells are formed.
Absorbed dose
Absorbed dose is the concentration of energy deposited in tissue as a result of an exposure to ionizing radiation. In civil defence terms, it means the energy absorbed by human tissue. In general only gamma radiation is considered for dosimetry purposes, although many of the instruments used are able to detect high energy beta radiation, alpha radiation is totally ignored except for the testing of food-stuffs and drinking water. Gamma-rays, unlike sunlight, can penetrate deep into the body and deposit energy in internal organs, they can even pass through a person's body. Absorbed dose describes the intensity of the energy deposited in any small amount of tissue located anywhere in the body.
For much of the Cold War period, the unit in use was the rad. The roentgen was commonly used for measuring the amount of ionization in the air caused by radioactive decay of nuclei for much of the Cold War. In non-bone biological tissue, one röntgen is the equivalent of about 0.93 rad. In air, one röntgen equals 0.87 rad. Dials that show calibration in mR/hr are reading milliröntgen per hour. The modern unit of measurement for absorbed dose is the milligray (mGy).
Physically speaking, the most elementary way to measure the effect of radiation is to measure the amount of energy deposited in a given weight of material. However, the deposition of energy is only one aspect of the potential of radiation to cause biological damage. The damage caused per unit of deposited energy is greater when it is deposited over a shorter distance. Hence an alpha particle, which would deposit its entire energy over a very short distance, causes far more damage per unit of energy than a gamma ray, which deposits its energy over a longer track. The weight of biological matter in which the energy is deposited is also important. The sensitivities of different organs also vary, the blood forming tissues, and the ovaries and testes are particularly sensitive. The concept of relative biological effectiveness (RBE) has been created to try to capture the relative efficiency of various kinds of radiation in producing biological damage.
Equivalent dose
Equivalent dose is an amount that takes the damaging properties of different types of radiation into account, as explained in the previous paragraph different types of radiation cause different amounts of damage. Absorbed dose tells us the amount of energy deposit in a volume of tissue. Equivalent dose addresses the impact that the type of radiation has on that tissue. The equivalent dose is measured in sieverts the equivalent dose for gamma radiation measured in milliSievert (mSv) = the absorbed dose in mGy.
Dose conversion factors (DCFs) are used to convert an amount of radioactivity (expressed in curies or becquerels) breathed or ingested by a person into a dose (expressed in rems and sieverts). The DCFs used for regulatory purposes are derived from a combination of a variety of experimental data and mathematical models.
Equivalent dose is calculated for individual organs. It is based on the absorbed dose to an organ, adjusted to account for the effectiveness of the type of radiation. Equivalent dose is expressed in millisieverts (mSv) to an organ.
Effective Dose
Effective dose is calculated for the whole body. It is the addition of equivalent doses to all organs, each adjusted to account for the sensitivity of the organ to radiation. Effective dose is expressed in millisieverts (mSv). Tissue weighting factors represent relative sensitivity of organs for developing cancer.
Half-life
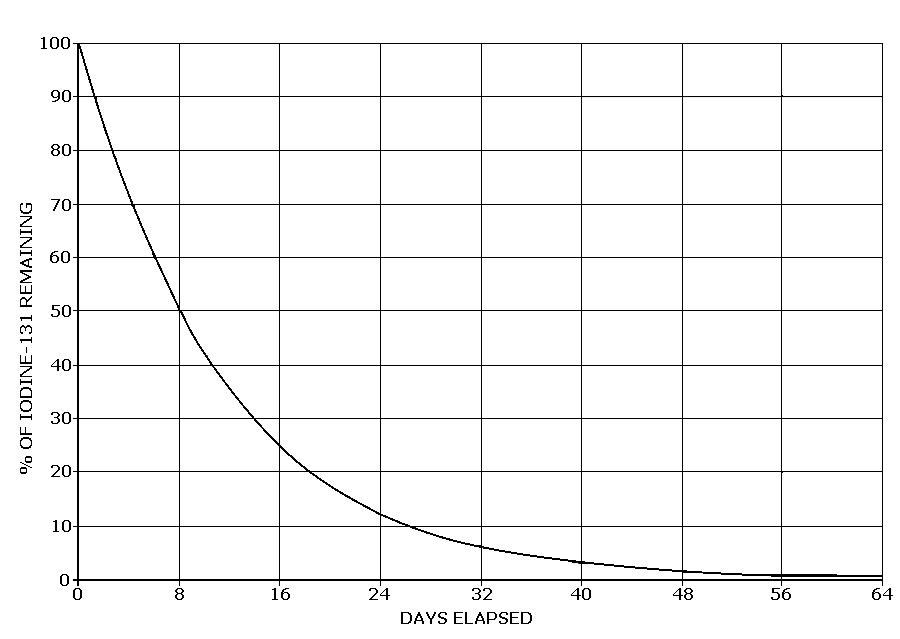
An isotope's half-life is, the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay, change spontaneously into other nuclear species by emitting particles and energy, or, equivalently, the time interval required for the number of disintegrations per second of a radioactive material to decrease by one-half.
The radioactive isotope 131iodine, which is a product of nuclear fission, has, for example, a half-life of 8 days. Thus after that interval, a sample originally containing 8 g of 131iodine would contain only 4 g of 131iodine and would emit only half as much radiation. After another interval of 8 days, the sample would contain only 2g of 131iodine.
Half-lives are characteristic properties of the various unstable atomic nuclei and the particular way in which they decay. Alpha and beta decay are generally slower processes than gamma decay. Half-lives for beta decay range upward from one-hundredth of a second and, for alpha decay, upward from about one one-millionth of a second. Half-lives for gamma decay may be too short to measure (around 10-14 second), though a wide range of half-lives for gamma emission has been reported.
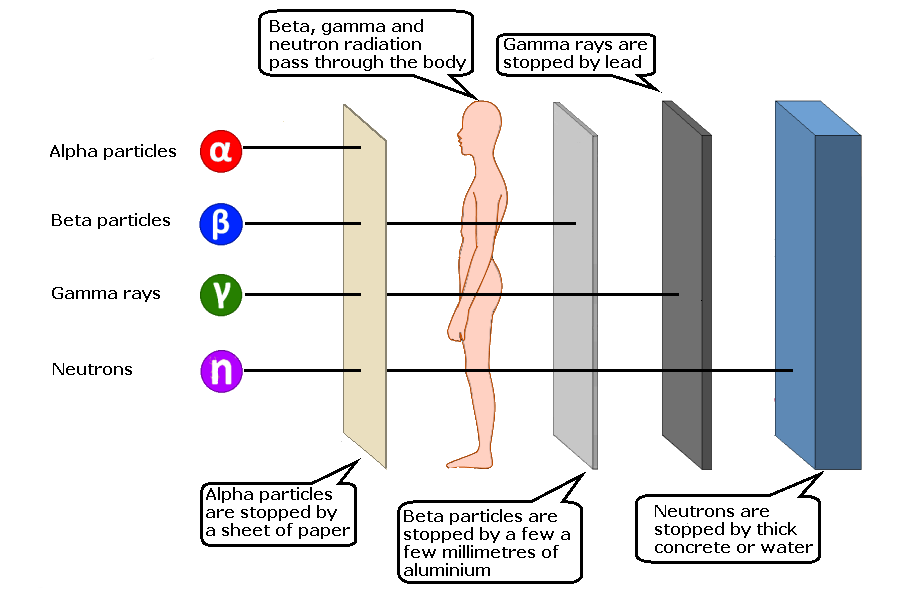
Penetrating power
Fundamentally the thicker and the more dense the material, the more readily it absorbs radiation. One of the basic principles of radiological protection is to get as great a thickness of dense materials between self and source as possible. A graphic showing relative penetration is shown to the right.Following a nuclear attack
By convention the nuclear radiation resultant from the detonation of a nuclear weapon is split between initial radiation and residual radiation.
Initial radiation
Initial radiation, also referred to as prompt radiation, by convention includes all nuclear radiation emitted in the first minute after detonation and the instantaneous radiation from the actual explosion. Initial radiation comprises neutrons, alpha particles, beta particles, positrons and gamma rays. Of these the alpha component can be effectively ignored, and at distances outside the zone of total destruction beta particles and positrons would not be an issue. For an air-burst, even at moderate height, the alpha and beta radiation will not reach the ground. What is of most significance is the highly penetrating gamma and neutron radiation.
Initial Gamma rays
The
detonation of a nuclear weapon releases an intense pulse of gamma
radiation. Gamma rays can penetrate a considerable thickness of
matter, e.g. the roof and walls of a building, but they are
attenuated or weakened in doing so: they can also be scattered back
from the atoms of oxygen and nitrogen in the atmosphere, causing an
additional hazard which has been described as invisible skyshine.
Skyshine is relatively diffuse and can come from any direction.
Protection behind a heavy obstacle in the line of sight only will
therefore not be so good as all-round cover under a heavy shield.
Gamma rays can penetrate the tissues of the body much more deeply
than alpha or beta particles; a high-energy gamma ray photon may, in
fact, pass through a human without interacting with tissue at all.
When gamma rays interact with tissue, they ionize atoms.
Initial neutrons
For
some distance around ground zero the neutron dose may he higher than
the gamma ?ash dose, but beyond a certain point the gamma hazard
predominates and this point is always well within the zone in which
strong blast and radiation protection are needed. It may therefore
happen that the neutron hazard is greater in shelters quite close to
the detonation of small tactical weapons with light cases which
permit a higher proportion of the neutrons to escape. Otherwise a
shelter which gives reasonable protection from gamma radiation will
also give good protection against neutrons.
Neutrons
which escape from the detonation are either captured immediately, or
are slowed down and then captured, by nuclei of neighbouring atoms.
When a neutron is captured by the nucleus of another atom the latter
becomes unstable and radioactive. This is called "induced" activity
and it will occur in the material underneath a ground or low air
burst and may be mixed with fall-out. Generally, activity induced in
the materials of the soil decays more rapidly than the average for
fission products and becomes insignificant within a few days.
Another, and particularly important form of induced activity with
immediate instead of prolonged e?fect, is the capture of neutrons by
the atoms of nitrogen in
the atmosphere, such atoms are intensely radioactive and promptly
emit an extremely penetrating gamma radiation, during their decay,
this intensities and extends the duration of the initial gamma
flash.
Residual radiation
Residual radiation comes from radioactive fission products from the nuclear reaction itself and from material rendered radioactive by the intense neutron bombardment that occurs during the explosion. These materials are initially in vapour form because of the extremely high temperatures involved, and they condense on particles of dust, falling to the ground as fall-out over a considerable area. The residual radiation decays rapidly at first, and then more slowly with time, and it may constitute a hazard for a considerable time.
Fallout
Radioactive fall-out is the radioactive material that falls from the atmosphere as a result of a nuclear explosion.
Fallout is a combination of three groups of materials: un-reacted material from the weapon itself, products of nuclear fission and/or fusion and material that has been rendered radioactive by neutron bombardment during the explosion. Principally the first group will be either 235U, 239Pu or tritium or a combination of them. The products of nuclear fission are varied, in the case of 235U, the most signifciant being isotopes of iodine, caesium, strontium, barium and xenon. The actual quantity of radioactive material in a nuclear weapon is relatively small, a few kilograms at most, and in the context of warfare the contamination they would produce may be discounted, of far more significance is the quantity of material produced as a result of neutron activation. A purely fusion weapon would produce virtually no fallout, however as all thermonuclear devices have a fission device at their heart the fallout generated is much the same in its properties.
To produce a contaminating burst, that is one where there is significant fallout, a weapon needs to be detonated at or near ground level. High level bursts produce relatively little fallout, and what there is is rapidly dispersed by upper atmosphere currents. The minimum heights for contaminating bursts are shown in the table.
Radioactive fallout has a physical appearance and behaviour similar to any other dust, with similar particle sizes and distribution. The time it takes to fall to earth depends upon the particle size and density, together with the distance it has to fall. Other factors which will affect the speed of deposition include wind speed and whether or not there is meteorological precipitation.
Generally speaking, fallout descends vertically, although initially it will have been spread by the force of the explosion itself. If a wind is blowing then it will travel laterally with the wind, forming what is known as a plume. The plume shown below is from the biggest test, Castle Bravo, conducted by the USA, a 15Mt thermonuclear device, probably one of the dirtiest ever detonated, being a fission-fusion-fission weapon. Wind was from the West predominantly, but with significant sheer at different altitudes. The quantity of fallout was far larger than expected, and the fireball much larger and higher, as the power of the bomb was far greater than had been predicted.
Neutron bombardment of non-nuclear components of weapons and of material exposed to the intense neutron bombardment resulting from the detonation as well as the fission process, produces hundreds of radioactive isotopes, well over 200 have been identified.
The un-fissioned material consists largely of alpha-emitters, whilst fission products and those produced as a result of neutron action are, in the main, beta and gamma emitters.
Fallout decay
Fortunately most of the products of nuclear explosions have very short half-lives ranging from nano-seconds to hours. Others have half-lives of anything upwards to thousands of years. The mean rate of radioactive decay for fallout products is pretty accurately known. Fallout decays according to what is known as the 7/10 rule. Given a level of fall-out one hour after a nuclear explosion, the level at seven hours will be 1/10th, after 49 hours it will be 1/100th. In the table shown the doserate at one and three-quarter hours after an explosion is given out of interest. Note that this rule applies to post detonation times, not fallout arrival or maximum times, both of which latter have been miss-used in a great deal of online content.
The above rule is based upon fallout that is primarily fission products. Neutron-induced radiation does not follow this rule. However, for planning purposes the rule holds good.
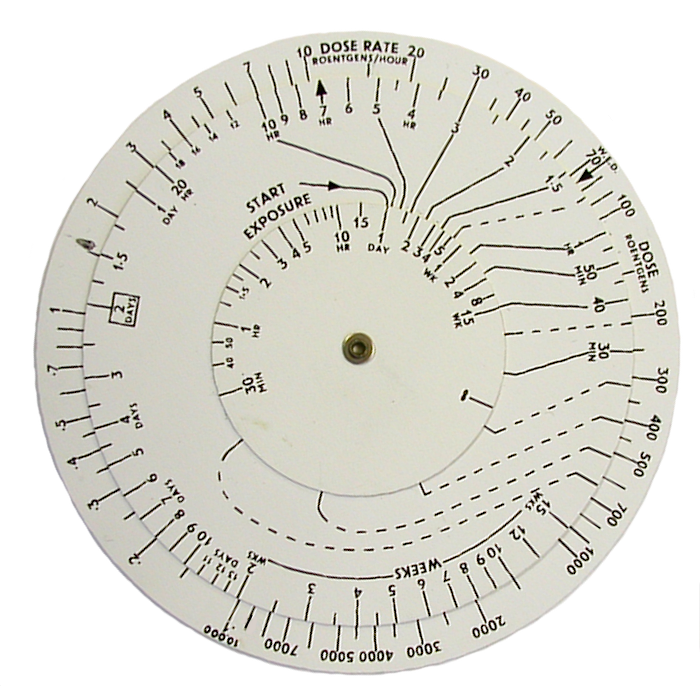
The rule may also be expressed mathematically as: I = I1*t-1.2, where I is the radiation dose rate at any time t, I1 the dose rate at unit time, and t the time measured from the instant of detonation.
In the UK, civil defence organisations were supplied with circular slide rule calculators to make the job of forecasting easier.
Radiation dose and health
Since the days of Marie Curie, it has been appreciated that ionising radiation exposure may be hazardous to health. The statutory limit, in the UK, on the amount of radiation to which the general public may be exposed in excess of natural background radiation and excluding medical exposure is set, from l January 2000, at l mSv per annum.
The most important source of man-made exposure, during peacetime, is medical investigation, which accounts for 90% of it. In the UK average natural background radiation is raised to 2.6 mSv by all man-made exposure. Other statutory limits include occupational dose limits. From 1 January 2000 these are 20 mSv per annum for classi?ed workers and 6 mSv per annum for unclassi?ed workers. The cold war Wartime Emergency Dose (WED) was set at 75 röntgen, equivalent to 750 mSv.
The biological effects of radiation can be classified as those due to short term exposure and those due to long-term exposure. In the short term low levels of radiation have relatively little effect. In the long term, continued exposure at low levels causes a significant increase in cancer risks and foetal abnormalities.
Weighting factors
Radiation damage to tissue and/or organs depends on the dose of radiation received, or the absorbed dose which is expressed in a unit called the gray (Gy). The potential damage from an absorbed dose depends on the type of radiation and the sensitivity of different tissues and organs. Some tissues are more susceptible to radiation damage than others, principally those where there is rapid cell generation, for example in the blood forming tissues, the lining of the gastrointestinal tract, the reproductive systems, and in cells involved with immune responses.
The effective dose is a measure of ionizing radiation in terms of the potential for causing harm. The sievert (Sv) takes into account the type of radiation and sensitivity of tissues and organs. It is a way to measure ionizing radiation in terms of the potential for causing harm.
The Sv is a very large unit so it is common to use smaller units such as millisieverts (mSv) or microsieverts (μSv). There are one thousand μSv to one mSv, and one thousand mSv to one Sv. In addition to the amount of radiation (dose), it is often useful to express the rate at which this dose is delivered (this is referred to as the dose rate), and is expressed as microsieverts per hour (μSv/hour) or millisieverts per year (mSv/year).
Beyond certain thresholds, radiation can impair the functioning of tissues and/or organs and can produce acute effects such as skin redness, hair loss, radiation burns, or acute radiation syndrome. These effects are more severe at higher doses and higher dose rates. The dose threshold for acute radiation syndrome is about 1 Sv (1,000 mSv).
If the radiation dose is low and/or it is delivered over a long period of time (low dose rate), the immediate effects are substantially less because there is a greater likelihood of repairing the damage. There is still a risk of long-term effects such as cancer, however, that may appear years or even decades later. Effects of this type will not always occur, but their likelihood is increased with accumulated radiation dose. This risk is higher for children and adolescents, as they are significantly more sensitive to radiation exposure than adults.
Epidemiological studies on populations exposed to radiation, such as atomic bomb survivors or radiotherapy patients, showed a significant increase of cancer risk at doses above 100 mSv. More recent studies have shown that there is a smaller, but significant increase in cancer risk in the 50-100 mSv exposure range.
Prenatal exposure to ionizing radiation may induce brain damage in foetuses following an acute dose exceeding 100 mSv between weeks 8-15 of pregnancy and 200 mSv between weeks 16-25 of pregnancy. Epidemiological studies indicate that the cancer risk after foetal exposure to radiation is similar to the risk after exposure in early childhood.
When radioactive material is ingested or breathed in the risks are considerably increased. Alpha emitters have relatively little effect when outside the body, but when in immediate contact with internal tissues cause considerable damage due to the intensity of the ionisation they cause.
Radiation protection:
A number of principles are involved in protection of the individual from the effects of radiation:
- Time: keep exposure times as short as possible, remembering that the effects of radiation are basically cumulative. Also decay of fallout means that at least in the early period following an explosion the decay of radiation is very rapid.
- Distance: Assuming a point source of radiation radiation decays according to Newton's inverse square law. This means that if we take a measurement at a distance of 1 metre from a point source, then at two metres the reading will be 25% , and at 4 metres only 6.25%. The actual reduction in intensity is slightly greater due to absorption/attenuation by the air.
- Density: The density of any intervening material between the source and the exposed person.Half value thicknesses
- Density and half values
- As mentioned above alpha and beta radiation do not penetrate to any distance. Gamma radiation reduces in intensity as it passes through materials, as its energy is transformed into heat. No material completely blocks all gamma radiation, no matter what its density or thickness. The actual, degree of attenuation depends upon the energy of the radiation, but the information here is based upon average values for fallout radiation. The degree of reduction or attenuation is proportional to the density of the material and its thickness. Dense materials absorb radiation more than less dense ones.
Half value thickness
Calculation of the degree of attenuation is based upon half-thicknesses. The half-thickness of a material is the thickness of the substance which will reduce gamma radiation by one half. It is important to note that if the radiation passes through another half-thickness of material it is not reduced to zero but by half again.
Protective factor (PF)
Using tables and measurements of thicknesses, together with structural details it is possible to estimate a protective factor (PF) for a building or other structure. A PF of 5,000 will give adequate protection from high levels of gamma radiation due to fallout. It is important to remember that the PF calculation also involves the physical distance from the fallout itself. A PF of 5,000 requires about twelve half-thicknesses. For dry earth this is about 1.3 metres.
Gamma radiation travels in straight lines, but like light, it can be scattered around sharp edges, such as the ends of walls, in a process known as diffraction. For a typical door sized opening, the angle of scatter is about 90°, and about 1 metre inside door-space, alongside the opening, the level of radiation would be reduced to about 1 - 2%. A winding entrance, or a long entrance tunnel or shaft would give a higher degree of protection than a simple door.